- home
- Our Science
- Exosomes
- Platform Technology
- Manufacturing
- SAB
- Publications
- Patent
Manufacturing
Pure-Exo®
Platform technology for high-purity
exosome manufacturing on
a commercial scale
Exosome Manufacturing for Therapeutic Application
The development of exosomes for therapeutic applications depends
on reproducible and analytical technologies
for the production and purification of clinical-grade exosomes which
can be applied on a commercial scale.

-
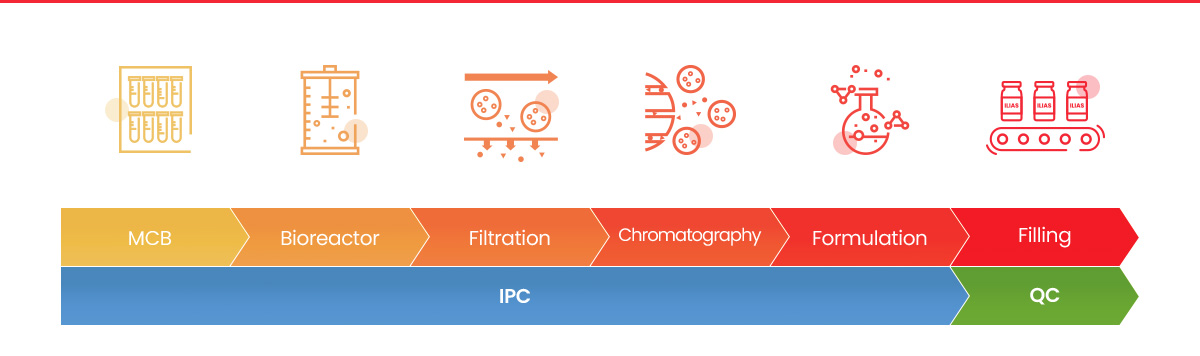
Master Cell Bank (MCB)
The success of the exosome manufacturing process starts with the development of a stable cell line for exosome
production. ILIAS establishes Master cell banks (MCB) and characterizes them thoroughly with high standards for
cGMP requirements. -
Cell Culture
ILIAS has successfully established scalable manufacturing processes using ILIAS’s bioreactor technology for suspension cell
culture to which ILIAS’ EXPLOR® technology can be applied. -
Filtration and Chromatography
Exosomes are isolated sequentially by ultrafiltration and chromatography to produce highly purified exosomes with
high yields. -
Formulation and Filling
Our exosome-based pharmaceuticals are properly formulated and packaged in GMP facilities to sustain stability.